
In order to trigger differentiation, colonies of human embryonic stem cells use dynamic molecular signaling waves that pass from cell to cell, researchers report.
Once prompted, the cells begin to organize into the three germ layers—the endoderm, mesoderm, and ectoderm—that ultimately become an embryo.
The discovery counters explanations dating back to 1952 research by British mathematician Alan Turing, who argued signaling gradients could self-organize through a mechanism now known as a Turing instability. Such a process would theoretically allow a stable gradient of molecules to deliver signals of different strengths to each cell.
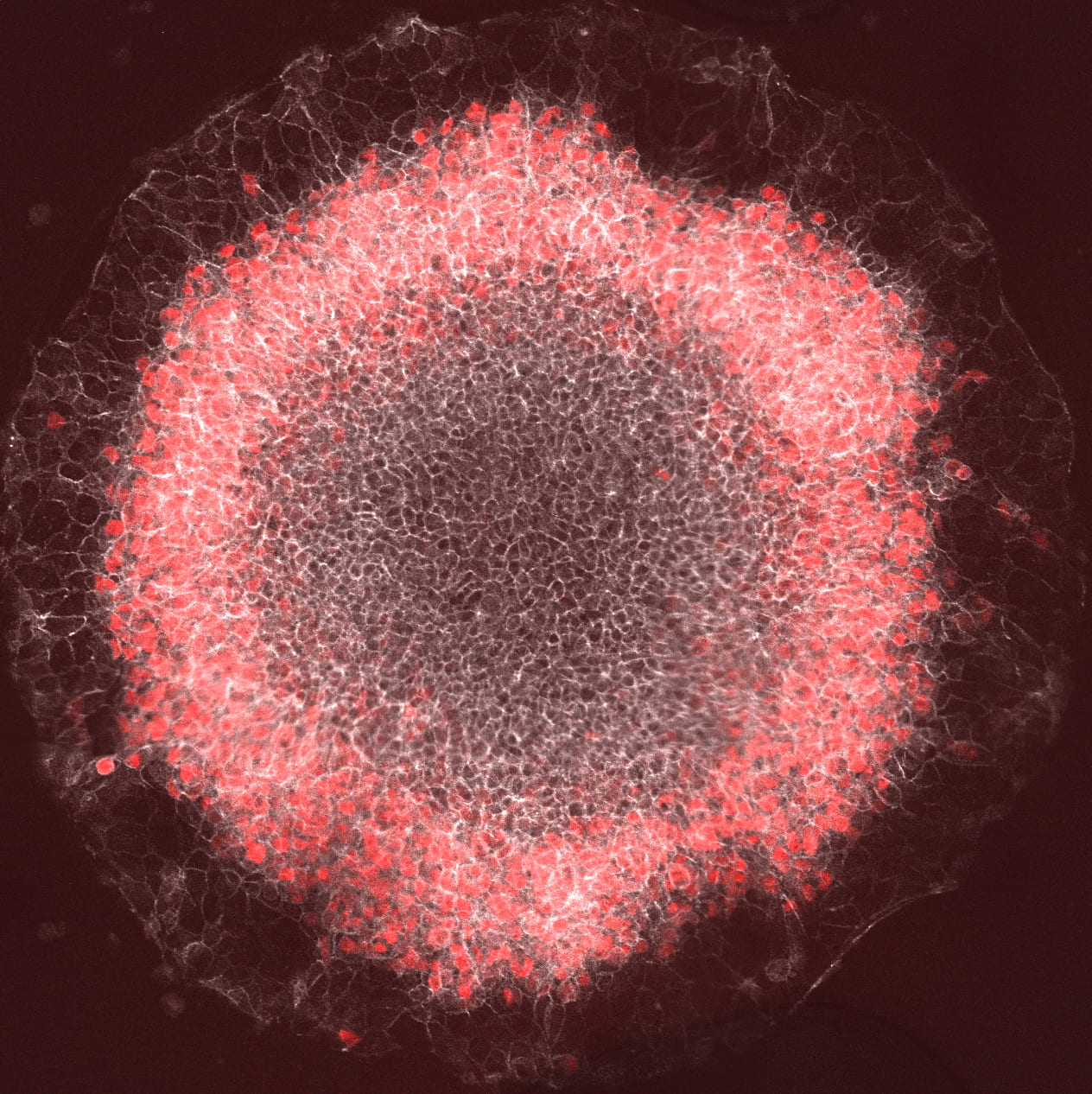
A stem-cell gastrulation model shows a wave of Wnt signals affecting bright cells. The black stripe-like regions in between cells are membranes that have been computationally removed from the images. (Credit: Warmflash lab/Rice)
The new research shows such gradients do not exist in colonies of embryonic stem cells and that the process is far more dynamic than previously appreciated. The researchers observed confined stem-cell colonies and used mathematical models to determine that such a mechanism could not explain the signaling patterns and waves they saw trigger differentiation into germ layers. These layers then turn into organs, bone, skin, and blood.
Catching the wave
The work, which appears in PLOS Biology, investigates dynamic interactions between the BMP, Wnt, and NODAL signaling pathways as part of a long-term study to decode the process by which a single fertilized cell becomes a human being. To do so, they use special patterned plates that force stem cells to grow in tiny circular colonies.
“We knew the important chemical signals but, until now, nobody has observed the activity of these signals in space and time.”
The researchers can then see, measure, and perturb the colonies as they progress through the very earliest stages, forming patterns of differentiated cells but never progressing to the point of becoming an embryo.
In the current study, the researchers applied BMP (bone morphogenetic protein) to the colonies. Signals transmitted through this pathway caused the cells to start and maintain a wave of cell-to-cell Wnt signals, which traveled from the perimeter toward the center of the colony.
Wnt, in turn, initiated a wave of NODAL signals that moved independently toward the center. By measuring the cascade, the researchers showed the duration of BMP signaling determined the position of the mesoderm—the middle layer in early embryo development—while Wnt and NODAL signals upregulated mesoderm differentiation.
Interactions between the signaling pathways determined where the mesoderm ring started and stopped, the researchers report.
“We knew the important chemical signals but, until now, nobody has observed the activity of these signals in space and time,” says lead author Sapna Chhabra, a graduate student at Rice University. “By focusing on the mesoderm, we showed that differentiation isn’t dependent on a particular level of any of the chemical signals the cells use.
“For now, we know that signaling starts at the colony edge and moves in, and that the position of red (stained mesoderm) cells correlates with where Wnt activity peaks,” she says. “There’s only a certain time period in which they can react.”
The fate of embryonic stem cells
Aryeh Warmflash, an assistant professor in the biosciences department, says the time it takes for signals to get to the center of the colony keeps them from differentiating as mesoderm as well.
“The signal is moving, continuously filling in the whole colony,” he says. “But depending on when it gets to particular cells, they either will or won’t respond. By the time the wave reaches them, cells at the center have already decided to become ectoderm.”
The researchers observed that the cells themselves migrate a little, but not nearly as fast as the fate-altering signals they pass along.
They also found that the cells at the perimeter of the colony were a good match for those known to become placental cells in the embryo, says Warmflash.
“There’s been quite a bit of controversy in the field over whether these cells represent placenta-like cells or not,” he says. “That’s because the decision to become a stem cell of the embryo itself or to become placenta should have happened before our whole model even starts.”
Chhabra examined the genes that the perimeter cells express by RNA sequencing and compared them to recently published sequencing data on placental cells from early human embryos.
“That allowed us, in this paper, to make the first comparison between those two things,” Warmflash says. “The bottom line is these cells are as good a model for the human placenta as the stem cells are for the human embryo. It’s not perfect, but it’s a reasonably good model.”
Additional coauthors are from Rice and Boston University. Rice, the Cancer Prevention and Research Institute of Texas, the Simons Foundation, and the National Science Foundation funded the research.
Source: Rice University
The post Embryo stem cells get marching orders via ‘waves’ appeared first on Futurity.
from Futurity https://ift.tt/32VIZfw
No comments:
Post a Comment